COVID-19
GenelQ utilizes real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing.
The Methodology
GenelQ utilizes real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing. Our COVID-19 testing methodology includes several assays that have received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA). These include the Lyra Direct Dry Swab assay from Quidel and the TaqPath COVID-19 Multiplex assay from ThermoFisher Scientific. By diversifying our COVID-19 assay portfolio, we have a steady supply of reagents and consumables to fulfill the demand for testing. We use leading ThermoFisher PCR instrumentation, which includes the QuantStudio 7Pro and the QuantStudio 12K Flex.
Sample Collection Methodology
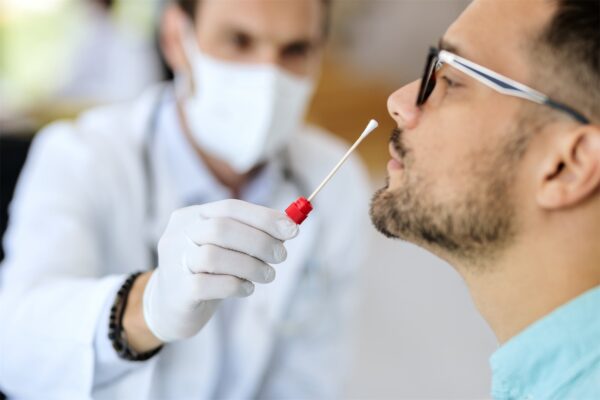
The GeneIQ sample collection methodology from Quidel employs a non-invasive, anterior nasal swab,which is inserted into the shallow portion of the nasal cavity. It is rotated four times along the anterior membrane of each nostril, and then placed in a collection tube for transport to the lab. Our physician-directed, self-administered sample collection method is as effective as the physician-administered nasopharyngeal swab, but without the discomfort.
Smart Service
At GeneIQ, we pride ourselves on providing high-touch, comprehensive service for supporting local and national clients. You can trust our team to provide exemplary customer service for your organization. We are always available to help streamline processes and provide a personalized solution. Our onboarding process, internet-based training and video conferencing-combined with our dedicated account management ensure a smooth implementation of our testing program.

Results Reporting
Test results are provided to the designated client coordinator via a Health Insurance Portability and Accountability Act (HIPAA)-compliant portal, as well as to the appropriate Centers for Disease Control and Prevention (CDC) office and state and local health administrative agencies.

Our Laboratory
GeneIQ utilizes real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing. Our COVID-19 testing methodology includes several assays that have received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA). These include the Lyra Direct Dry Swab assay from Quidel and the TaqPath COVID-19 assay portfolio. We use leading ThermoFisher PCR instrumentation, which includes the QuantStudio 7Pro and the Quant Studio 12K Flex. In addition our unique method for viral RNA extraction is fast and efficient, further increasing our capacity for throughput while minimizing dependence on the short supply of plastic consumables.
We also offer Antigen Testing
Our SARS Antigen Test is a lateral flow immunoassay that allows for the rapid, qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in anterior nares (NS) swab specimens directly from individuals who are suspected of COVID-19 by their healthcare provider. This test is intended for use within the first five days of the onset of symptoms or for individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. Our SARS Antigen Test provides accurate and reliable results in 10 minutes.
-
PPA (Reference Extracted SARS-COV-2 RT-PCR Assay)
96.6% -
NPA (Reference Extracted SARS-COV-2 RT-PCR Assay)
99.3%
COVID PLUS
This multiplex approach eliminates the need for multiple tests or doctor visits so patients can quickly get on the right treatment plan post onset of symptoms.

A 3-in-1 Advantage
Our COVIDPLUS test is the same premium PCR test as our standalone COVID test, but with a 3-in-1 advantage. This multiplex panel enables healthcare providers to quickly and accurately diagnose between the three major respiratory viruses.
This multiplex approach eliminates the need for multiple tests or doctor visits so patients can quickly get on the right treatment plan post onset of symptoms.
How much is the out of pocket (no insurance) price for PCR Covid-19 testing?
$200 PCR test for Covid-19
Vaccinations
GeneIQ Vaccine Direct, a division of GeneIQ, is committed to supporting your organization with zero cost on-site COVID-19 vaccinations.
Why GeneIQ?
GeneIQ has been a trusted resource throughout the pandemic and has serviced over half a million patients. With over 50 nurses on staff, our GeneIQ Vaccine Direct division is DSHS approved to administer Moderna, Pfizer, and Johnson and Johnson vaccines for COVID-19. We supply all of the medical staff and supplies on-site with end-to-end service at NO COST to the patient or organization. We also offer boosters and flu vaccines via a safe and convenient process.
Schedule Your AppointmentI am more than pleased to have this opportunity to celebrate a successful collaboration between Richardson ISD and GeneIQ. Working through the pandemic over the course of the last 18 months has definitely had its challenges. Fortunately, working with GeneIQ to vaccinate over 700 of our students was not one of them. When GeneIQ reached out to RISD, I immediately had confidence that they would do everything in their power to deliver a successful outcome. Here are just few highlights of our experience:
- Collaborative and organized
- Immediate response to email and phone calls
- Quick adjustments and willingness to pivot upon request
- Reflective
- Supportive and responsive to community
- Friendly and supportive team
RISD looks forward to opportunities to collaborate with GeneIQ in the future. They are definitely on the top of our partner list.
Brenda Payne
Assistant Superintendent
Administrative Services - Richardson Independent School District
Put Us to the Test
- Give Us a Call 972-942-0110
- Send Us an Email info@geneiqlab.com

